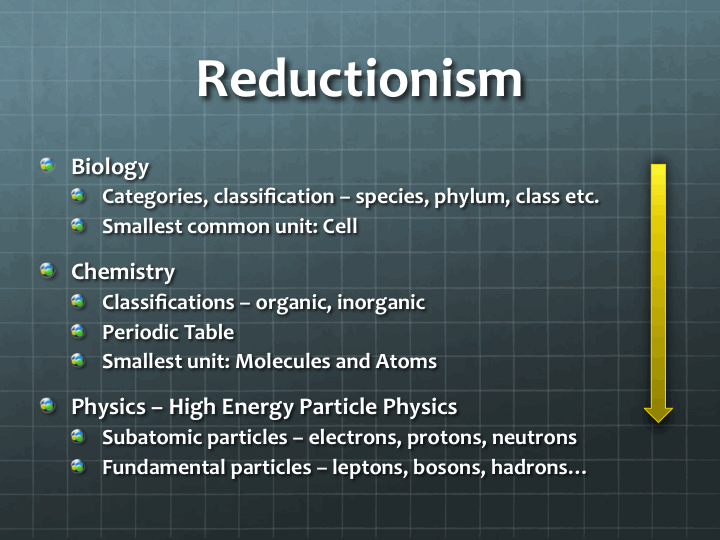
In all our scientific endeavors, we use similar high-level techniques to understand and study things. The most common technique is reductionism. It is based on the belief that the behavior, properties and structure of large and complex objects can be understood in terms of their simpler constituents. In other words, we try to understand the whole (the universe, for instance) in terms of smaller, reduced constituents (such as particles).
Classification vs. Division
Reductionism takes us in two distinct tracks. Firstly, among collections of objects or entities, we try to find commonalities by categorizing and classifying them so that we need to study only one sample to understand the whole. The second track is in looking at physically smaller and smaller sub-structures until we cannot go any further without destroying the essential properties of the structure under our scrutiny. The smallest such sub-structure is what we will call the smallest unit. We will see examples of these two approaches, namely classification and division, in the discussion that follows.
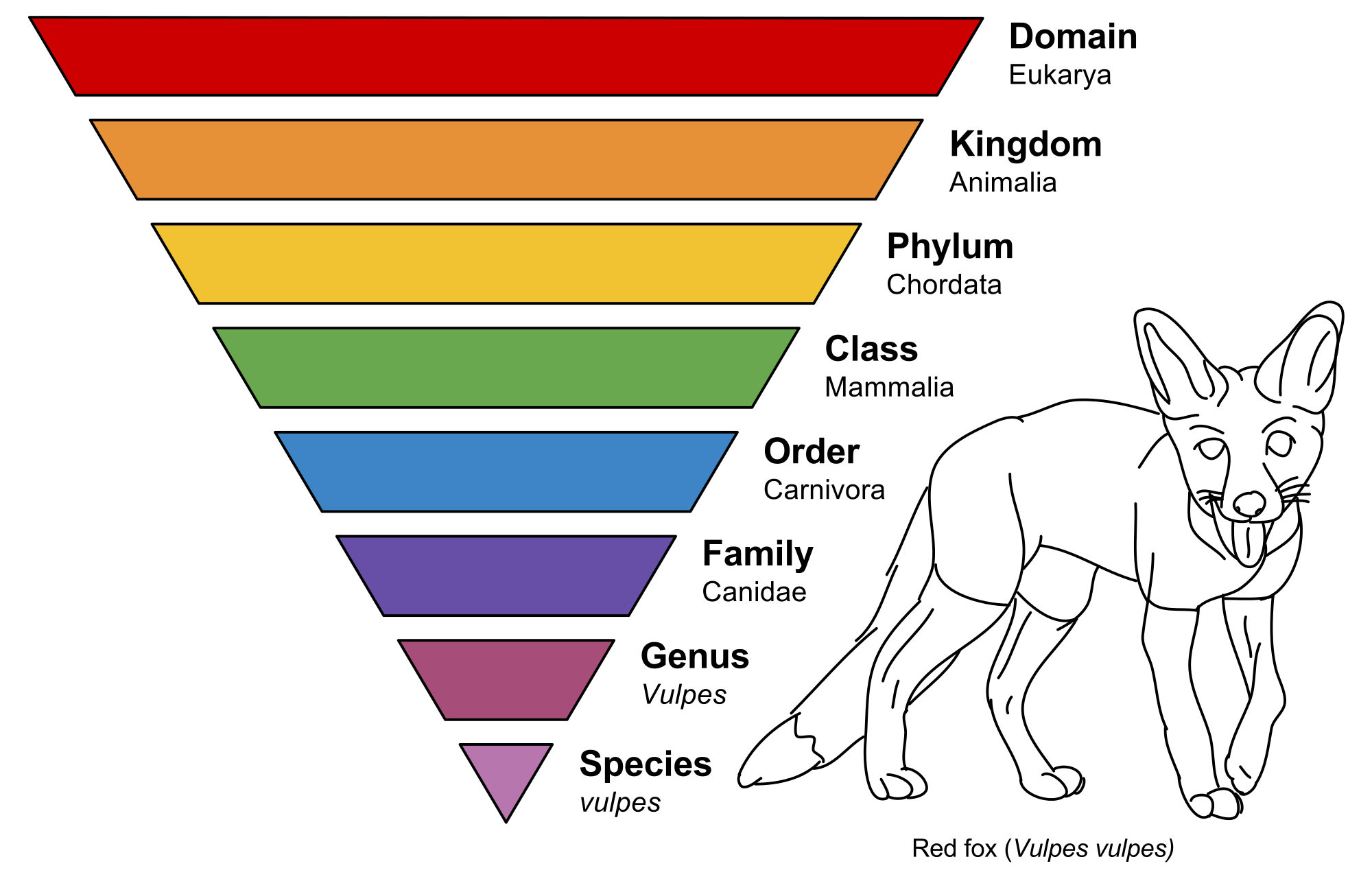
Figure 2. Classification scheme in biology, illustrated with a red fox.
In biology, reductionism of the first kind gives us the phylum-class-genus-species taxonomy scheme (see Figure 2 above), which has proven to be extremely effective. The second kind of reductionism is in trying to find the smallest unit the can be studied, which a biologist will tell you is a cell.
In chemistry, the smallest unit is a molecule or an atom. At the same time, chemists have robust classification schemes. The periodic table of elements is one. Other schemes follow categories such as organic, inorganic, metallic, non-metallic, acids, alkaline and so on.
In physics, especially the physics we are talking about, we go much smaller than atoms, following the second track of reductionism. But when we run into a large number of particles, we do use the first track (classification) as well – we divide the subatomic particles into categories (with some overlapping ones) such as leptons, quarks, bosons, hadron, baryons, fermions etc. The smallest units among them are considered fundamental particles. Since the smallest unit in particle physics is smaller than that of all other sciences, we could consider physics the most fundamental of them all.
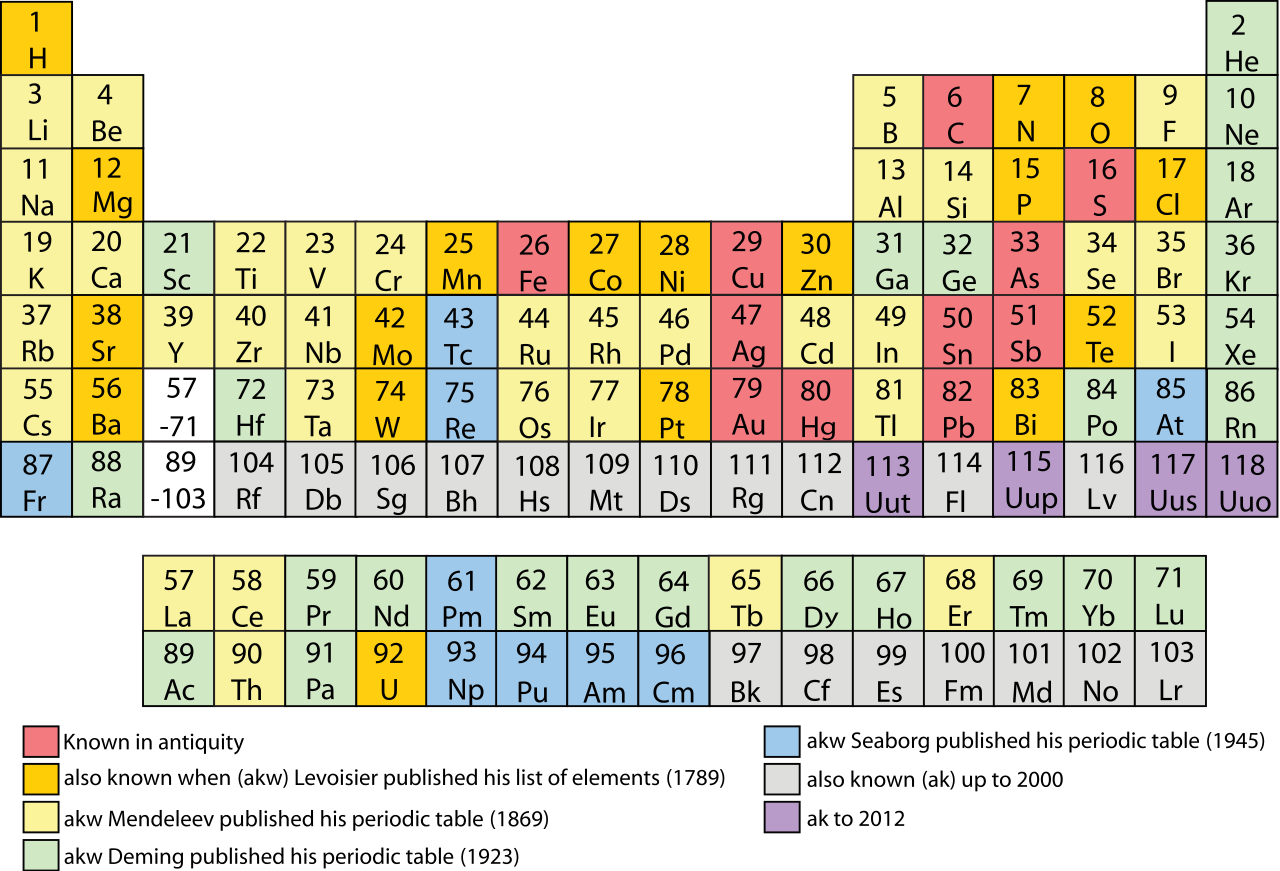
Figure 3. The most recognizable classification scheme in chemistry – the periodical take of elements.
Smallest Unit
Let me spend a few moments on what I mean by “the smallest unit” really is. If you take a cell in biology, it can function as a living thing. If you break it up, you get some large molecules. However, it won’t be a living thing any more. In that sense, biology doesn’t apply to the broken parts (except that molecular biology might). This is why I call cell the smallest unit in biology.
Similarly in chemistry, if you take a molecule like H2O, for instance, it has certain properties. If you break it up, you get hydrogen and oxygen, with vastly different properties. If you take a hydrogen atom and separate its electron from the proton, what you get is not even matter as we know it. The particles you get are neither elements nor compounds. As a result, chemistry no longer applies to them. Thus, atoms are the smallest units in chemistry.
In fact, the physics that applies to the particles (quantum mechanics) is very different from the one that applies to macroscopic objects. It is a mistake to think of an electron (for example) as a tiny point particle of matter. An electron has an existence only in terms of some numbers and equations that we can solve and experimentally verify. Its reality is a philosophical stance known as scientific realism, but that may be more philosophy than you want from this write-up.
Limitations of Reductionism
Although it serves as extremely well in our sciences, reductionism has its limits. It may not work in certain cases. Take an H2O molecule for instance. Is it wet or dry? What is its temperature? These questions make sense only in the context of statistical collections of molecules. So there is something that we lose as we apply our reductionist methods to matter. There is a transition in properties when you go from large collections of molecules to individual ones.
Similar phase transitions occur when collections of large molecules become alive and form cells, or when collections of live cells develop consciousness and the ability to “know”. What we know, of course, is only a reflection or projection of what is out there in the physical world into our conscious awareness. Viewed in this light, physics doesn’t subsume anything; it is biology with its capacity for perception and cognition that subsumes everything else. It is important to keep this perspective also in mind, because physicists often tend to forget it.